|
Alchimia has chosen to carry out biological analyses, in addition to the chemical ones, on its products to offer greater guarantees and respond to the growing need to have increasingly controlled and safe products on the market.
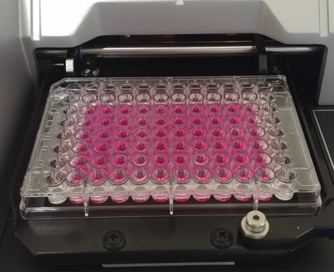
The biological cytotoxicity tests that we perform on the BALB 3T3 and ARPE-19 cell lines are part of the large family of biocompatibility tests for the biological evaluation of medical devices according to ISO 10993-1. Specifically, the product to be tested is placed in contact with an appropriate cell population. The toxicity is determined quantitatively (cell mortality) and morphological (change in the shape of the cells). The test is carried out in compliance with the ISO 10993-5 standard and validated methods to offer greater guarantees on product safety. |
|
Privacy Notice
|
© M.I.S.S Ophthalmics Ltd
|